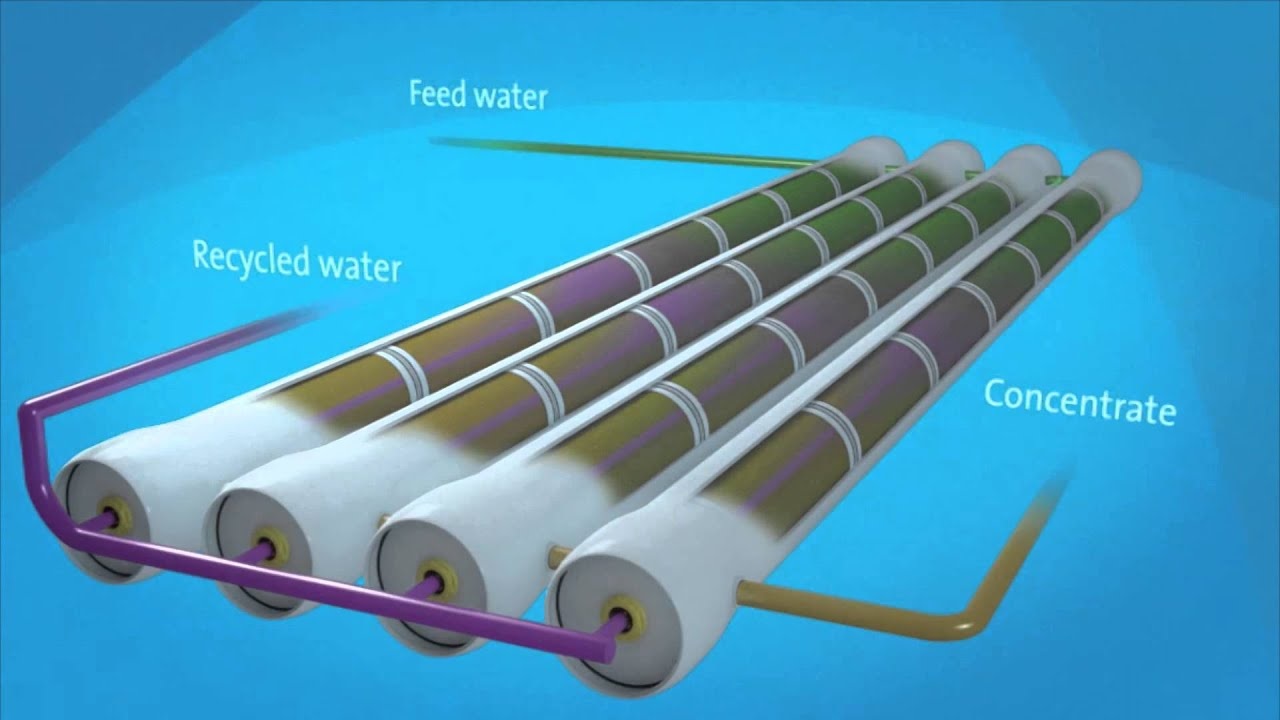
Reverse Osmosis, often abbreviated to RO, is a process that is used to purify water and make it fit for human consumption. This is done with the help of a semipermeable membrane (also known as a partially permeable membrane) which has the ability to filter out the relatively large particulate matter and unwanted ions/molecules from the water. A brief description of the reverse osmosis process is provided in this article.
What is Osmosis?
Understanding of the process of osmosis is a prerequisite for understanding the process of reverse osmosis. Osmosis refers to the movement of solvent molecules from a region where the solute concentration is low to a region where the concentration of solute particles is high. These solvent particles pass through a semipermeable membrane in order to achieve this movement.
The pressure that must be applied on the solution side of the semipermeable membrane in order to halt the flow of solvent particles is referred to as the osmotic pressure.
What is the Process Behind Reverse Osmosis?
When pressure (of a magnitude which is greater than the osmotic pressure) is applied on the side of the semipermeable membrane in which the solute concentration is high, the flow of the solvent particles through the semipermeable membrane is reversed. The solvent particles now move through the semipermeable membrane to the side in which the concentration of the solute particles is low.
Osmotic pressure must not be confused with critical pressure. Critical pressure is the pressure that must be applied in order to liquefy a substance at its critical temperature. Osmotic pressure is the pressure that must be applied to the solution side of the semipermeable membrane in order to halt the process of osmosis. To learn more about reverse osmosis and the working of a reverse osmosis plant, subscribe to the BYJU’S YouTube channel and enable notifications.